argon valence shell|Khan Academy : iloilo Argon Valence Electrons | Argon Valency (Ar) with Dot Diagram
Current time zone for Melbourne, Australia is AEST, whose offset is GMT+10. DST starts on Sun, October 6 2024 at 2:00 am local time, when time in Melbourne moves forward 1 hour to 3:00 am. DST ends on Sun, April 6 2025 at 3:00 am local time, when time in Melbourne moves back 1 hour to 2:00 am.
PH0 · Valences of the Chemical Elements
PH1 · Valence electron
PH2 · Valence Electrons Chart for All Elements
PH3 · Khan Academy
PH4 · How Many Valence Electrons Does Argon (Ar) Have?
PH5 · Argon Valence Electrons (And How to Find them?)
PH6 · Argon Valence Electrons
PH7 · 6.4 Electronic Structure of Atoms (Electron Configurations)
PH8 · 3.7: Electrons and Valence Shells
#CitizenTV #citizendigital
argon valence shell*******119 rows — Mar 23, 2023 — For main group elements (i.e s-block and p-block elements), the valence electrons are the electrons present in the outermost orbit. But for most of the transition and inner transition elements, the valence .Hul 20, 2023 — Group 18 elements (helium, neon, and argon are shown in Figure 2) have a full outer, or valence, shell. A full valence shell is the most stable electron configuration. Elements .argon valence shell Khan AcademyMar 20, 2023 — The total number of electrons in the last shell after the electron configuration of argon is called the valence electrons of argon. The last shell of argon has eight electrons. .How Many Valence Electrons Does Argon (Ar) Have?How Many Valence Electrons Does Argon (Ar) Have? [Valency of Ar]
Argon Valence Electrons | Argon Valency (Ar) with Dot Diagram3.7: Electrons and Valence Shells - Chemistry LibreTexts93 rows — This table of element valences includes the maximum valence and most common .Khanmigo is now free for all US educators! Plan lessons, develop exit tickets, and so much more with our AI teaching assistant. Get it now!Ene 24, 2021 — Well, Argon has an absolute zero valency. Argon holds 18 electrons in its outer shell with the electron configuration of 2,8,8. So, this is why Argon has no need to either gain .
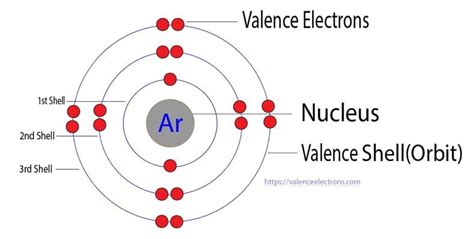
Ene 14, 2021 — Noble gases like argon have eight valence electrons so it does not require to lose or gain electrons to complete its energy shell i.e. stable octet. So that they do not have any tendency to combine with other elements which .

The valence shell is the set of orbitals which are energetically accessible for accepting electrons to form chemical bonds. For main-group elements, the valence shell consists of the ns and np .
The electrons occupying the outermost shell orbital(s) (highest value of n) are called valence electrons, and those occupying the inner shell orbitals are called core electrons (Figure 6.28). .argon valence shellMay 25, 2023 — Argon has 8 valence electrons because there are 8 electrons present in the outermost shell of the Argon (Ar) atom. Now let’s see how you can easily find the valence .
Okt 8, 2020 — The electron configuration of argon(Ar) shows that the last shell of argon(Ar) has eight(3s23p6) electrons. Therefore, the valence electrons of argon(Ar) are eight. What is the atomic number of an argon atom?Mar 22, 2023 — Note 1: If you want the valence electrons of all the 118 elements, then visit this article: Valence electrons chart for ALL ELEMENTS (Where I have shown the valence electrons using images). Note 2: If you want a periodic table with valence electrons labeled on it, then visit this article: Periodic table with Valence electrons (labeled on it) (From this article, you can also .Abr 16, 2024 — If an atom has 4 electrons in its valence shell , then it has an equal chance of gaining, losing or sharing it's valence electrons in order to achieve octet. . Hence, Valency of Argon is 0 and it is called inert gas or .Mar 17, 2023 — The atomic number of argon is 18. That is, the number of electrons in argon is eighteen. Therefore, the argon atom will have two electrons in the first shell, eight in the 2nd orbit, and eight electrons in the 3rd shell. Therefore, the order of the number of electrons in each shell of the argon(Ar) atom is 2, 8, 8.Mar 20, 2023 — Step-3: Determine the valence shell and calculate the total electrons. The third step is to diagnose the valence shell. The last shell after the electron configuration is called the valence shell. . The inert gases of Group-18 are helium(He), neon(Ne), argon(Ar), krypton(Kr), xenon(Xe), and radon(Rn). We know that the element in group-18 is .
Ago 16, 2024 — argon valency, electronic configuration, atomic number and shell
Photoionization of rare gas clusters in the innervalence shell region has been investigated using threshold photoelectron and photoion spectrometers and synchrotron radiation. Two classes of states are found to play an important role: (A) valence states, correlated to dissociation limits involving an ion with a hole in its innervalence ns shell, (B) Rydberg states correlated to .Mar 20, 2023 — The total number of electrons in a valence shell is called valence electrons. However, the valence electrons of the transition elements are located in the inner orbit. . (Ti 4+) has acquired the electron configuration of argon and it achieves an octave full stable electron configuration. That is, in this case, the valency of the titanium ion .
Mar 20, 2023 — The 26th element in the periodic table is iron. The 1 st element in group 8 is iron and its symbol is ‘Fe’. The elements in groups 3-12 are called transition elements. The valence electrons are the total number of electrons in the last orbit (shell). But in the case of transition elements, the valence electrons remain in the inner shell (orbit).
Mar 23, 2023 — Electron shell arrangement; 1: Electron configuration of Hydrogen (H) 1s 1: 1s 1: 1: 2: Electron configuration of Helium (He) 1s 2: 1s 2: 2: 3: . Electron configuration of Argon (Ar) [Ne] 3s 2 3p 6: 1s 2 2s 2 2p 6 3s 2 3p 6: 2, 8, 8: 19: Electron configuration of Potassium (K) . Periodic table with Valence Electrons Labeled (7 HD Images .Nob 28, 2023 — Why does argon have 18 electrons Argon: argon valence electron configuration Krypton diagram copper structure atomic dot electron argon atom shell atoms lewis label svg file configuration model valence selenium draw. Argon: Argon Valence Electron Configuration. Electron argon bohr calcium diagram model configurations configuration silicon .Khan AcademyArgon has 26 known isotopes, from 29Ar to 54Ar and 1 isomer (32mAr), of which three are stable (36Ar, 38Ar, and 40Ar). Argon-40 is composed of 18 protons, 22 neutrons, and 18 electrons. . The number of electrons in each element’s .Dis 9, 2019 — Statement-2 : Argon is inert towards chemical reactivity due to the completely filled valence shell electronic configuration, high ionization enthalpy and more positive electron gain enthalpy. A. Statement-1 is True, Statement-2 is True,Statement-2 is a correct explanation for .Ago 6, 2024 — The outermost shell comprises 5 electrons i.e. it has 5 valence electrons. 4. Argon. The argon's atomic Number is 18. Electronic configuration: $1{s^2}2{s^2}2{p^6}3{s^2}3{p^6}$. The outermost shell comprises 6 electrons, and it is also fully filled with an orbital. The argon, then, is a noble gas. 5. Arsenic. Arsenic atomic number is 33.In argon, K, L, and M shells are filled. Hence, argon has 8 valence electrons. Explanation of incorrect option: Option (D): This is the invalid option. As in the outermost shell, 8 maximum number of electrons can be accommodated. Option (B): Oxygen, sulphur, etc. contains the 6 valence electron, not argon.Set 4, 2022 — The full valence shell means, the valence shell is fully occupied and forms a stable octet. The halogens like Helium, Neon, Argon, Xenon, etc. Why is the valence shell so important in studying chemical reactions? Because when two atoms interact, the electrons in the outermost shells are the first ones to come into contact with each other and .Okt 12, 2023 — The number of valence electrons available for the Argon atom is 8. Argon is situated in Group 18th or 8A and has an atomic number of 18. The first shell of Argon has 2 electrons and the outer shell or valence shell of Argon has 8 electrons, hence, the number of valence electrons in the Argon atom is 8.argon valency, electronic configuration, atomic number and shell
We would like to show you a description here but the site won’t allow us.All the winning numbers about 2 tarikh ka lottery sambad fax are posted online, and you can also view the sambad lottery night 2 tarik 8 pm result below. . 29 tarik result, the winner’s list, and any old results for every date, ensuring you’re always up to date on the lottery’s past. Nagaland Lottery Sambad 29 Tarik Morning 1 PM Result .
argon valence shell|Khan Academy